Lijst met elementen
»
actinium
»
argon
»
astatine
»
barium
»
bismut
»
bohrium
»
Borium
»
broom
»
cadmium
»
calcium
»
cerium
»
cesium
»
chloor
»
chromium
»
curium
»
dubnium
»
erbium
»
europium
»
fermium
»
fluorine
»
fosfor
»
francium
»
gallium
»
goud
»
hafnium
»
hassium
»
helium
»
holmium
»
ijzer
»
indium
»
iridium
»
jodium
»
kalium
»
kobalt
»
koolstof
»
koperen
»
krypton
»
lantaan
»
lithium
»
Lood
»
lutecium
»
mangaan
»
natrium
»
neon
»
Nihonium
»
nikkel
»
niobium
»
nobelium
»
osmium
»
platina
»
polonium
»
radium
»
radon
»
rhenium
»
rhodium
»
rubidium
»
rutenium
»
samarium
»
scandium
»
selenium
»
silicium
»
stikstof
»
tallium
»
tantalum
»
Tennesse
»
terbium
»
thorium
»
thulium
»
tin
»
titanium
»
uranium
»
vanadium
»
wolfraam
»
xenon
»
yttrium
»
Zilver
»
zink
»
zuurstof
»
zwavel
mineralogie
elementen
V vanadium
V - vanadium - METAALACTINIDEN
Vanadium is een chemisch element uit de familie van overgangsmetalen, met het symbool V en atoomnummer 23. Het is een dicht en relatief kneedbaar zilvergrijs massief metaal met een relatief hoge hardheid en dichtheid. Het heeft een zeer hoog smeltpunt en lost langzaam op in water, maar kan worden opgelost met behulp van chemicaliën.
vanadium is het op vier na meest voorkomende element in de aardkorst, maar het is zelden in bruikbare hoeveelheden aanwezig en wordt vaak geassocieerd met andere elementen. Het werd in 1830 ontdekt door de Zweedse chemicus Nils Sefström.
vanadium wordt veel gebruikt in verschillende industriële en commerciële toepassingen, vooral in de lucht- en ruimtevaartindustrie en raffinage. Het wordt ook gebruikt als katalysator in de chemische industrie en als additief in staal om hun weerstand en hardheid te verbeteren. In zijn natuurlijke staat is vanadium giftig en mag het niet worden ingeademd of ingeslikt. Recente studies tonen aan dat vanadium kan helpen het cholesterolgehalte te verlagen en de cardiovasculaire gezondheid te verbeteren.
vanadium is een belangrijk element voor de vervaardiging en het gebruik van brandstofbatterijen. Het wordt ook gebruikt bij de productie van legeringen voor gereedschappen en vuurwapens en voor het maken van kleurstoffen. Andere toepassingen zijn fotosynthese, de productie van corrosieremmers en de productie van katalysatoren voor diesel- en benzinemotoren.
vanadium is het op vier na meest voorkomende element in de aardkorst, maar het is zelden in bruikbare hoeveelheden aanwezig en wordt vaak geassocieerd met andere elementen. Het werd in 1830 ontdekt door de Zweedse chemicus Nils Sefström.
vanadium wordt veel gebruikt in verschillende industriële en commerciële toepassingen, vooral in de lucht- en ruimtevaartindustrie en raffinage. Het wordt ook gebruikt als katalysator in de chemische industrie en als additief in staal om hun weerstand en hardheid te verbeteren. In zijn natuurlijke staat is vanadium giftig en mag het niet worden ingeademd of ingeslikt. Recente studies tonen aan dat vanadium kan helpen het cholesterolgehalte te verlagen en de cardiovasculaire gezondheid te verbeteren.
vanadium is een belangrijk element voor de vervaardiging en het gebruik van brandstofbatterijen. Het wordt ook gebruikt bij de productie van legeringen voor gereedschappen en vuurwapens en voor het maken van kleurstoffen. Andere toepassingen zijn fotosynthese, de productie van corrosieremmers en de productie van katalysatoren voor diesel- en benzinemotoren.
Synthetisch
Radioactief
Vloeistof
Gasvormig
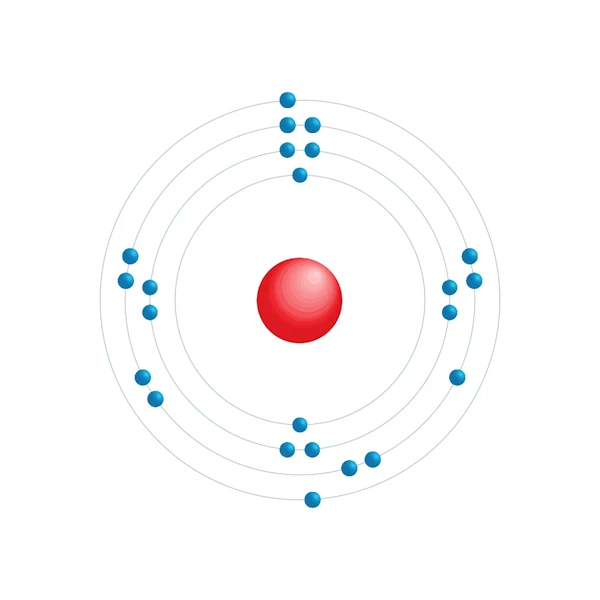
Elektronisch configuratiediagram
| Namn | vanadium |
| Aantal | 23 |
| Atomair | 50.9415 |
| Symbool | V |
| Fusie | 1890 |
| Koken | 3380 |
| Dichtheid | 6.11 |
| Periode | 4 |
| Groep | 5 |
| Ontdekking | 1801 del Rio |
| Overvloed | 120 |
| Straal | 1.9 |
| Elektronegativiteit | 1.63 |
| Ionisatie | 6.7462 |
| Aantal isotopen | 9 |
| Elektronische configuratie | [Ar] 3d3 4s2 |
| Oxidatie stelt | -1,2,3,4 |
| Elektron op energieniveau | 2,8,11,2 |
| Mineralen | Hardheid | Dichtheid |
| Allendeite | 4.84 | |
| Alvanite | 3.00 / 3.50 | 2.41 |
| Andreyivanovite | ||
| Ankangite | 6.50 / 6.50 | 4.44 |
| Ankinovichite | 2.50 / 3.00 | 2.48 |
| Anorthominasragrite | 1.00 / 1.00 | 2.12 |
| Ansermetite | 3.00 / 3.00 | 2.57 |
| Aradite | ||
| Ardennite-(As) | 6.00 / 7.00 | 3.62 |
| Ardennite-(V) | 6.00 / 7.00 | 3.55 |
| Argandite | ||
| Averievite | 4.00 / 4.00 | 3.54 |
| Balestraite | 2.50 / 3.00 | 2.95 |
| Bannermanite | 3.50 | |
| Bariandite | 2.70 | |
| Bariosincosite | 3.00 / 3.00 | |
| Barnesite | 3.00 / 3.00 | 3.15 |
| Bassoite | 4.00 / 4.50 | 2.94 |
| Batisivite | 7.00 / 7.00 | 4.62 |
| Bavsiite | ||
| Beckettite | ||
| Berdesinskiite | 6.00 / 6.50 | |
| Blossite | 3.95 | |
| Bluestreakite | 2.00 / 2.00 | 2.63 |
| Bobjonesite | 1.00 / 1.00 | 2.28 |
| Bokite | 3.00 / 3.00 | 2.97 |
| Borisenkoite | ||
| Brackebuschite | 4.00 / 5.00 | 6.05 |
| Burnettite | ||
| Bushmakinite | 3.00 / 3.50 | 6.22 |
| Byrudite | 7.00 / 7.00 | 4.35 |
| Calciodelrioite | 2.50 / 2.50 | 2.45 |
| Calderónite | 3.00 / 4.00 | 6.05 |
| Calvertite | 4.00 / 5.00 | 5.24 |
| Carnotite | 2.00 / 2.00 | 3.70 |
| Cassagnaite | ||
| Cassedanneite | 3.50 / 3.50 | 6.00 |
| Cavansite | 3.00 / 4.00 | 2.21 |
| Cavoite | ||
| Cechite | 4.50 / 5.00 | 5.88 |
| Cheremnykhite | 5.50 / 5.50 | 6.00 |
| Chernykhite | 3.00 / 4.00 | 3.14 |
| Chervetite | 2.00 / 2.50 | 6.30 |
| Cleusonite | 6.00 / 7.00 | 4.74 |
| Clinobisvanite | 6.95 | |
| Cloncurryite | 2.00 / 2.00 | |
| Colimaite | 2.24 | |
| Colusite | 3.00 / 4.00 | 4.20 |
| Coparsite | ||
| Coronadite | 4.50 / 5.00 | 5.44 |
| Cortesognoite | ||
| Corvusite | 2.50 / 3.00 | 2.82 |
| Coulsonite | 4.50 / 5.00 | 5.17 |
| Crichtonite | 5.00 / 6.00 | 4.46 |
| Curienite | 3.00 / 3.00 | 4.88 |
| Davidite-(Ce) | 6.00 / 6.00 | 4.42 |
| Davidite-(La) | 6.00 / 6.00 | 4.42 |
| Davisite | ||
| Delrioite | 2.00 / 2.00 | 3.10 |
| Descloizite | 3.50 / 3.50 | 6.10 |
| Dessauite-(Y) | 6.50 / 7.00 | 4.68 |
| Dickthomssenite | 2.50 / 2.50 | 1.96 |
| Dissakisite-(La) | 6.50 / 7.00 | 3.79 |
| Doloresite | 3.27 | |
| Dreyerite | 2.00 / 3.00 | 6.25 |
| Dugganite | 3.00 / 3.00 | 6.33 |
| Duttonite | 2.50 / 2.50 | 3.24 |
| Engelhauptite | 3.86 | |
| Erlianite | 3.50 / 3.50 | 3.11 |
| Evdokimovite | ||
| Fernandinite | 2.00 / 3.00 | 2.78 |
| Ferribushmakinite | 2.00 / 2.00 | 6.15 |
| Fervanite | 3.28 | |
| Fianelite | 3.00 / 3.00 | 3.21 |
| Fingerite | ||
| Francevillite | 3.00 / 3.00 | 4.55 |
| Franciscanite | 4.00 / 4.00 | 4.00 |
| Fritzscheite | 2.00 / 3.00 | 4.39 |
| Gamagarite | 4.50 / 5.00 | 4.62 |
| Gatewayite | ||
| Germanocolusite | 4.50 / 5.00 | 4.00 |
| Goldmanite | 6.00 / 7.00 | 3.74 |
| Goldquarryite | 3.00 / 4.00 | 2.78 |
| Gottlobite | 4.50 / 4.50 | 3.41 |
| Gramaccioliite-(Y) | 6.00 / 6.00 | 4.66 |
| Grantsite | 1.00 / 2.00 | 2.94 |
| Greenwoodite | 5.00 / 5.00 | 4.81 |
| Grigorievite | 5.00 / 5.00 | 3.67 |
| Gunterite | 1.00 / 1.00 | 2.40 |
| Gurimite | ||
| Häggite | 4.50 / 4.50 | 3.00 |
| Hanjiangite | 4.00 / 4.00 | 3.78 |
| Haradaite | 4.50 / 4.50 | 3.80 |
| Hechtsbergite | 4.50 / 4.50 | 6.87 |
| Hemloite | 6.50 / 6.50 | 4.00 |
| Hendersonite | 2.50 / 2.50 | 2.77 |
| Hereroite | 8.15 | |
| Hewettite | 2.50 | |
| Heyite | 4.00 / 4.00 | 6.30 |
 mineraly.fr
mineraly.fr
 mineraly.co.uk
mineraly.co.uk
 mineraly.com.de
mineraly.com.de
 mineraly.it
mineraly.it
 mineraly.es
mineraly.es
 mineraly.nl
mineraly.nl
 mineraly.pt
mineraly.pt
 mineraly.se
mineraly.se